Medicare, a government-based health insurance program for seniors, was established by Congress in 1965. While the program has evolved over the past 57 years, there have been no significant changes recently for the simple reason that any changes to Medicare require bipartisan support, and that is a rarity these days in Congress.
While Medicare provides excellent coverage for hospitalization, outpatient procedures, and physician’s services, additional benefits like dental, vision, and hearing have only been available to veterans or through programs like Medicare Advantage or Medicaid. One of the reasons for this is the strong, effective lobbying from groups like the American Dental Association, who have pushed hard against adding dental coverage to the basic Medicare package of benefits.
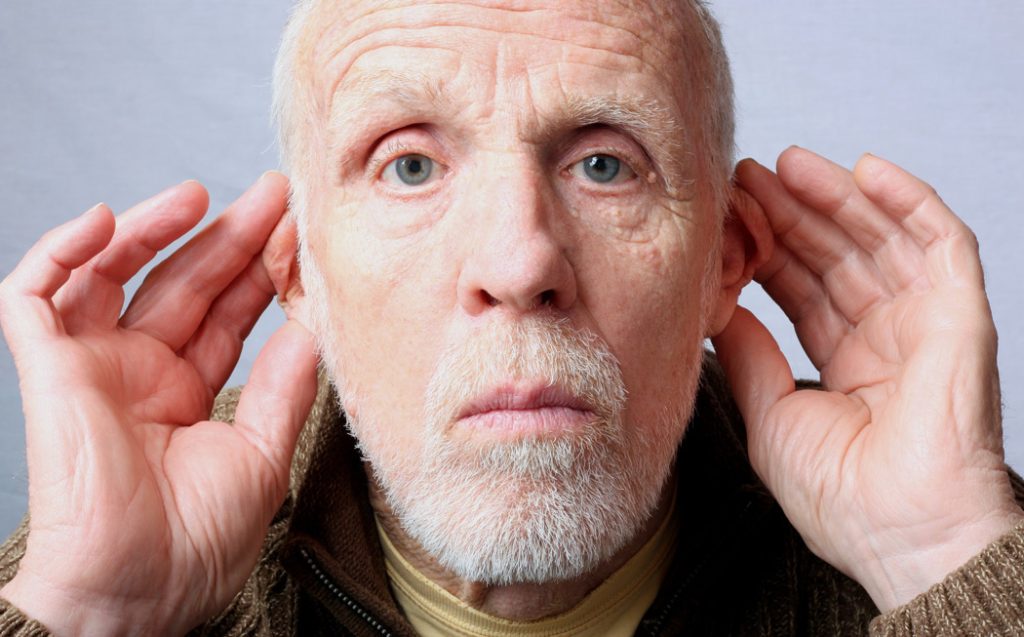
While most seniors can manage the cost of dental cleanings and fillings, it’s a different story when it comes to hearing aids. About one-third of Americans between the ages of 65 and 74 have lost at least some of their hearing, and after 75 that rises to nearly half. Most of these people don’t get hearing aids. In fact, among those with hearing loss age 70 or older, less than one-third have ever even tried hearing aids. Hearing deficits are horribly isolating for the elderly. Unaddressed, they can accelerate cognitive deterioration. Some of these older people may be stubborn or in denial, but the biggest obstacle is cost. Hearing aids are very expensive for most seniors on a fixed income, with a pair of hearing aids typically costing between $4,000 and $6,000.
Congress finally did something about this in 2017 when it passed a bipartisan bill allowing for the over-the-counter sale of hearing aids, which, thanks to the advent of smartphones and other advances in consumer electronics, will cost perhaps as little as one-tenth as much as prescription-based hearing aids. The “Over-the-Counter Hearing Aid Act” required the Food and Drug Administration to create a new category of hearing aids that would meet high standards but also provide consumers with the option of a regulated, FDA-approved device at lower cost. Under this new category, consumers with “mild to moderate” hearing loss would also be able to purchase this device without a prescription or exam through in-person transactions, mail, or online. For adjustments, people would not need to visit an audiologist; with over-the-counter hearing aids, they could just use an app on their smartphone for tweaking their devices.
Although this bill was signed into law by then-President Trump, the FDA waited over four years to issue regulations for this new category of hearing aids. In October 2021, the FDA finally announced the new regulations, kicking off a four-month public comment period. During that period, the Big Five hearing aid companies launched a massive counterattack, fabricating hundreds of fake public comment letters designed to influence the FDA to make changes that would benefit the hearing aid manufacturers, reduce the competition, and increase costs to consumers. The Big Five also put pressure on the FDA to strike the words “moderate” from the proposal, hoping to limit those eligible to purchase the new hearing aids to those people with only “mild” hearing loss. All told, the Big Five spent over a million dollars lobbying in 2022. Their efforts were largely unsuccessful.
The prolonged FDA delay resulted in several companies jumping onboard the OTC hearing aid market without waiting for FDA approval. Leading consumer brands Bose and Apple for example, are offering over-the-counter hearing aid products. And Apple last year integrated hearing assistance into its popular AirPods Pro earbuds, which operate more as a sound amplification device than a hearing aid.
Finally in July 2022, President Biden cut through the red tape and delays and issued an Executive Order requiring the FDA to take definitive action on over-the-counter hearing aids within 120 days. This led to the FDA issuing the final guidelines for OTC hearing aids on August 16, 2022. This final ruling incorporated several changes from the earlier proposal, including lowering the maximum sound output to reduce the risk of hearing damage from overamplification of sound, revising the insertion depth level in the ear canal, and requiring that all OTC hearing aids have a user-adjustable volume control.
While it will likely be next summer before consumers see the new FDA approved devices arrive in the marketplace, seniors on Medicare can take some satisfaction in knowing that, for once, this battle was won for the common good.
Nikki Weaver is the owner of Heartland Insurance Solutions of Southeast Iowa. She is a licensed insurance agent specializing in Medicare.
